When you pick up a prescription at your local pharmacy, you might not think twice if the bottle says lisinopril instead of Zestril. But behind that simple swap is a whole different system than what happens in a hospital pharmacy. Retail and hospital pharmacies don’t just fill prescriptions differently-they operate under completely separate rules, goals, and processes when it comes to swapping one drug for another. Understanding these differences isn’t just for pharmacists. It affects your safety, your bills, and even whether your treatment works as intended.
What Gets Substituted-and Who Decides
In retail pharmacies, substitution is mostly about generics. If your doctor prescribes a brand-name drug like Lexapro, the pharmacist can usually switch it to escitalopram without asking anyone-unless the doctor checks "do not substitute" or you say no. This is legal in all 50 states, and it happens in about 90% of eligible cases. The main driver? Cost. Insurance companies push for generics because they save money-often 80% less than brand names. Retail pharmacists don’t need approval from a doctor. They make the call at the counter, based on state laws and formulary rules. Hospital pharmacies work differently. There’s no quick swap at the window. Instead, a team of pharmacists, doctors, and nurses meets regularly on a Pharmacy and Therapeutics (P&T) committee to decide which drugs can be swapped across entire units. If a hospital wants to switch from vancomycin to linezolid for MRSA infections, they don’t just change the label. They review clinical data, check for side effects, train staff, update electronic records, and notify every doctor who might prescribe it. This isn’t about saving money alone-it’s about improving safety and following clinical pathways.Types of Drugs That Can Be Swapped
Retail substitution is mostly limited to pills and capsules. Over 97% of substitutions in community pharmacies are for oral solid dosage forms. You won’t see a pharmacist swapping your IV antibiotic or your insulin pen at the drive-thru. Why? Because those drugs are complex, sensitive, and often require special handling. Insurance formularies also rarely cover substitutions for specialty drugs-only about 13% of them are even eligible. In hospitals, substitution goes far beyond pills. Nearly 70% of therapeutic interchanges involve IV medications, biologics, or compounded formulas. A patient on Remicade (a biologic for Crohn’s disease) might be switched to a biosimilar like Inflectra if the P&T committee approves it. That kind of decision requires deep clinical knowledge. It’s not just about price-it’s about how the drug behaves in the body, how it’s administered, and whether the patient has already had a reaction to the original.Who Gets Notified-and How
At the retail level, you’re the one who gets told. Forty-seven states require pharmacists to inform you when a substitution happens. Some give you a printed notice. Others ask you verbally. It’s your right to say no. But here’s the catch: many patients don’t remember the conversation, or they assume the pharmacist knows best. A 2023 Consumer Reports survey found that 14% of patients were confused about why their medication looked different-some even thought it was a mistake or a counterfeit. In hospitals, it’s the doctor who gets notified-not the patient. When a therapeutic interchange happens, the electronic health record updates automatically. The prescribing physician gets an alert within 24 hours. The pharmacist doesn’t wait for consent-they follow the hospital’s approved protocol. This isn’t about bypassing the doctor. It’s about integrating the change into the patient’s entire care plan. If a patient is on a new heart failure regimen, the nurse, the cardiologist, and the pharmacist all need to be on the same page.
Why the Differences Matter for Patient Safety
The biggest risk isn’t in the substitution itself-it’s in the handoff between settings. When a patient leaves the hospital and goes home, their discharge summary might say they’re on metoprolol tartrate. But their retail pharmacy, following insurance rules, dispenses metoprolol succinate. These are different formulations. One is taken twice daily. The other is extended-release and taken once. Mix them up, and the patient could get too much or too little medication. The Institute for Safe Medication Practices found that nearly 24% of medication errors during hospital-to-home transitions involve substitution mismatches. That’s not because pharmacists are careless. It’s because the systems don’t talk to each other. One system uses formulary codes. The other uses clinical names. One tracks substitution history. The other doesn’t. This is why new rules are coming. Starting in July 2024, CMS requires all insurers and providers to share standardized substitution records during care transitions. Hospitals and retail chains are slowly starting to connect their systems. Epic and Cerner are building tools to show a patient’s full substitution history-whether it happened in the ER, the ICU, or your local CVS.What Pharmacists Experience Daily
Retail pharmacists spend hours fighting insurance companies. One pharmacist on Reddit described calling three times just to get prior authorization for a generic that insurance refused to cover-even though it was cheaper. They’re caught between patients who want the brand name because "my doctor said it’s better," and insurers who demand the cheapest option. About 64% of retail pharmacists say prior authorization delays are their biggest headache. Hospital pharmacists deal with something else: physician resistance. Even when a P&T committee approves a switch to a safer, cheaper drug, some doctors refuse to change their habits. One hospital pharmacist shared that it took six months and 15 education sessions to get cardiology to adopt a new beta-blocker protocol. Their challenge isn’t paperwork-it’s changing clinical culture. But hospital pharmacists also see results. After switching to a standardized antibiotic protocol, one hospital cut C. difficile infections by 38%. That’s not just cost savings-it’s lives saved.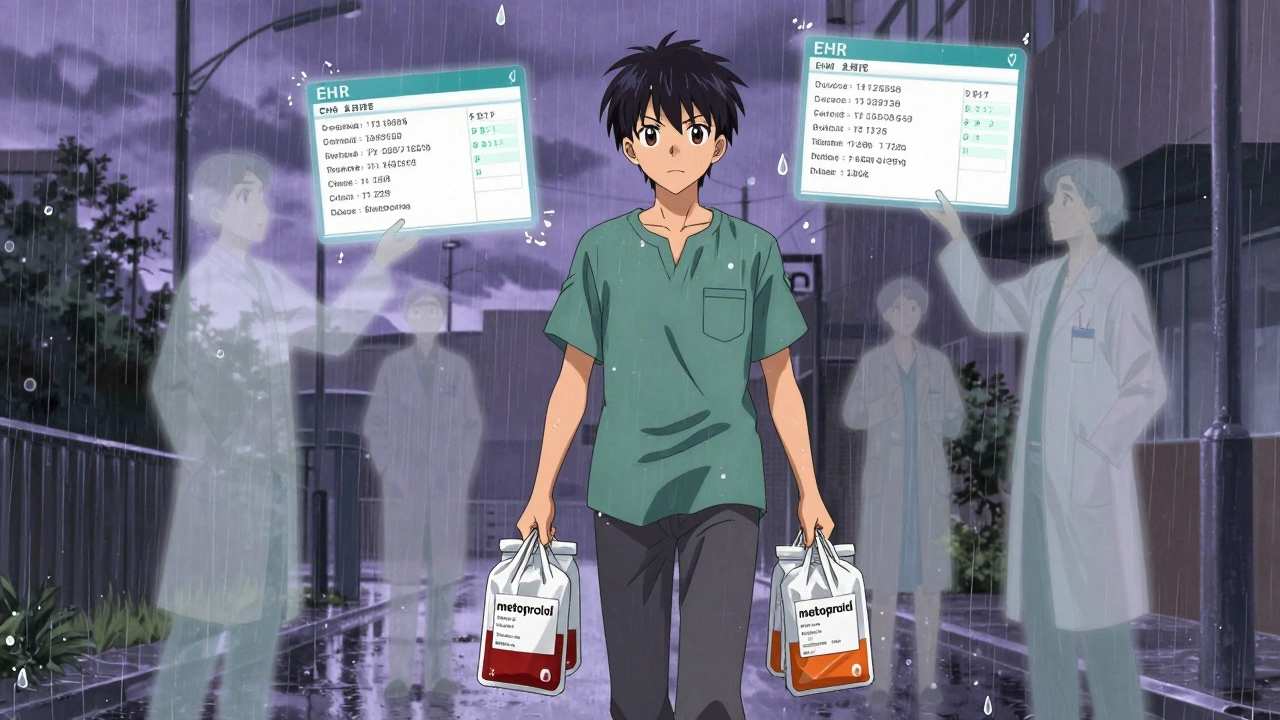
What’s Changing-and What’s Not
Retail substitution will always be about saving money. The Generic Pharmaceutical Association estimates generic drugs saved the U.S. healthcare system $317 billion in 2023 alone. That’s not going away. But the way it’s managed is evolving. More retail chains are now offering discharge follow-up programs. If you’re discharged from the hospital with a new prescription, a pharmacist might call you a few days later to make sure you got the right drug and understand how to take it. Hospitals are also feeling the pressure to cut costs. With the 340B Drug Pricing Program, many hospitals are using substitution to stretch their budgets. But they’re doing it smarter-linking every swap to clinical outcomes, not just price tags. The future isn’t about one system replacing the other. It’s about connecting them. By 2028, experts predict 78% of healthcare systems will have unified substitution protocols. That means when you get your prescription filled, whether it’s at the hospital or your neighborhood pharmacy, the system will know what you’ve been switched to-and why.What You Need to Know
If you’re on a long-term medication, always ask: "Was this drug changed? Why?" Keep a list of every medication you take-including the name, dose, and form (immediate-release, extended-release, etc.). Bring that list to every appointment, whether you’re seeing your primary care doctor or a specialist. Don’t assume a generic is the same as the brand. Some drugs, like thyroid medication or seizure drugs, need to be taken exactly as prescribed. Even small changes can make a difference. If you’re discharged from the hospital, ask the pharmacist: "Did anything change while I was in?" Then check your prescription again at the retail pharmacy. If it looks different, ask why. The system isn’t perfect. But awareness is your best defense.Can a retail pharmacist refuse to substitute a brand-name drug with a generic?
Yes. While pharmacists are allowed to substitute generics under state law, they must honor any "do not substitute" notation from the prescriber. Patients can also refuse substitution at the counter. In 32 states, pharmacists must verbally inform you of the substitution, and 18 states require written consent for the first-time switch. If you’re unsure, always ask.
Why can’t hospitals just use retail-style substitution?
Hospital patients are often sicker, on multiple medications, and at higher risk for adverse reactions. Substituting a drug without clinical review could cause harm. For example, swapping one blood thinner for another might seem simple-but if the patient has kidney issues or is on other drugs, the interaction could be dangerous. Hospital substitution requires team-based approval, documentation in the electronic record, and physician notification. Retail substitution is transactional. Hospital substitution is clinical.
Are biosimilars substituted the same way in retail and hospital settings?
No. Retail substitution of biosimilars (like switching from Remicade to Inflectra) is governed by state laws, and only 23 states have passed specific biosimilar substitution rules as of 2023. Some require pharmacist notification, others require prescriber consent. In hospitals, biosimilar switches are handled through P&T committee protocols, with clinical data reviewed and physician alerts triggered. The rules are stricter in hospitals because biosimilars are used for serious conditions like cancer and autoimmune diseases.
What’s the biggest danger when switching between retail and hospital pharmacies?
The biggest danger is mismatched formulations. For example, a patient might be discharged on metoprolol succinate (once-daily extended-release), but the retail pharmacy dispenses metoprolol tartrate (twice-daily immediate-release). This can lead to under- or overdosing. These errors happen in nearly a quarter of hospital-to-home transitions. Always compare your discharge instructions with your retail prescription-and ask if anything changed.
Will substitution practices become the same in retail and hospital pharmacies in the future?
They’re moving toward alignment, but they won’t become identical. Retail will keep focusing on cost savings and patient choice. Hospitals will keep focusing on clinical safety and team-based decisions. What’s changing is the connection between them. New EHR systems being rolled out by 2025 will share substitution history between settings. This means your hospital pharmacist and your retail pharmacist will both see what you were switched to-and why-reducing errors during transitions.

Lawrence Armstrong
December 12, 2025 AT 00:11Been a pharmacist for 12 years. Retail = cost control. Hospital = safety first. Both are necessary, but the handoff? Total nightmare. Saw a guy get double-dosed because his discharge med was extended-release and the retail pharmacy gave him immediate-release. He ended up in the ER. 😔
sandeep sanigarapu
December 13, 2025 AT 09:45This is very important information. Many patients do not understand the difference between generic and brand. In India, we also face similar issues. Always check the medicine name and form. Safety first.
Robert Webb
December 13, 2025 AT 18:25Let me just say this: the real problem isn't substitution itself-it's the lack of communication between systems. Retail pharmacies operate on a transactional model: fill the script, collect the copay, move on. Hospitals operate on a clinical model: assess, consult, document, monitor. When these two worlds collide-like when a patient gets discharged and walks into CVS-the system doesn't just fail, it actively misleads. The fact that 24% of transition errors involve formulation mismatches isn't a glitch-it's a design flaw. We're treating medication like a commodity instead of a clinical intervention. And until we start treating the EHRs like integrated medical records instead of disconnected billing tools, this will keep happening. We need interoperability standards that include substitution history, not just drug names. And yes, I know that sounds like a pipe dream… but so did electronic prescribing 20 years ago.
Adam Everitt
December 13, 2025 AT 21:21so like… retail = cheap, hospital = careful? yeah i get it. but why cant they just… i dunno… share data? like, real time? im not a doc but even i know this sounds like a dumb system. lol. also, metoprolol tartrate vs succinate?? i thought they were the same. guess not. 🤯
wendy b
December 15, 2025 AT 11:23Actually, this entire piece is quite superficial. You mention biosimilars but fail to address the pharmacokinetic variability that makes substitution in autoimmune patients statistically dangerous without therapeutic drug monitoring. Also, the 90% substitution rate in retail? That’s a product of insurance coercion, not clinical wisdom. And you call it ‘patient safety’? Please. The real issue is the erosion of prescriber autonomy under corporate formularies. This isn’t healthcare-it’s actuarial science with a white coat.
Audrey Crothers
December 15, 2025 AT 19:39OMG YES THIS!! I had a friend get switched from one beta-blocker to another after hospital discharge and she ended up with crazy dizziness and heart palpitations… turns out the pharmacy gave her the wrong formulation. She didn’t even know to ask! 😭 We need mandatory pharmacist follow-ups after hospital discharge. Like, a phone call. Just one. It could save lives. 🙏
Reshma Sinha
December 16, 2025 AT 11:25From a clinical pharmacy standpoint, the P&T committee model in hospitals is the gold standard. Therapeutic interchange isn't just about cost-it's about standardization, evidence-based protocols, and reducing practice variation. The fact that retail pharmacies can swap a 10mg lisinopril for a 20mg without clinician input is a regulatory gap waiting to explode. We need a tiered substitution framework: low-risk meds = automatic; high-risk = clinician-triggered. And biosimilars? They need a 30-day observation window post-switch. Period.
Donna Anderson
December 17, 2025 AT 03:43i just got my meds switched at the pharmacy and didn't even notice till i read this. my blood pressure meds look different now. i thought they just changed the bottle. 🤦♀️ i'm calling my doc tomorrow. thanks for the heads up!!